Huge discounts for flexible research raises concerns about future epidemics

Public health experts have warned that the decision of health and humanitarian services (HHS) Robert F. Kennedy Junior this week’s resolution to cancel hundreds of millions of dollars in financing a flexible vaccine that will leave the United States not ready for the next epidemic and other public health emergencies.
“I tried to be objective and unanimous in response to the current HHS behavior-but frankly, this step will cost life,” said the wounds of former President Trump, Jerome Adams, in a position on the social platform x.
“Merna technology is used that exceeds vaccines … and the vaccine that they helped develop in record time is due to the provision of millions.”
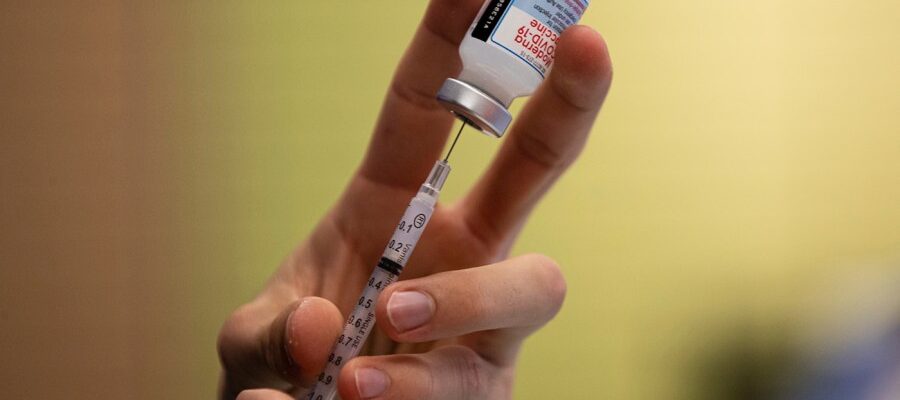
The first Covid-19 MRNA vaccines from Pfizer and Moderna achieved the market in 2021, just one year after the virus appeared for the first time. The vaccines usually take years to develop, but flexible shots have been developed in record time thanks to a huge flow of financing from the first Trump administration, which is called the warp.
Covid shots have proven safe and effective, and they helped end the epidemic. Experts say that a flexible technique has the ability to revolutionize treatments for advanced pathogens, especially bird flu, because the platform can be easily adjusted.
On Tuesday, Kennedy canceled contracts worth $ 500 million related to the research of a biomedical research and medical development authority (Barda).
Instead, he said that the agency will focus on platforms with “strongest safety records”.
Public health experts indicate that the reviews of hundreds of millions, if not billions, for doses of flexible shots that are managed all over the world have found very few negative events.
“It is quite clear that the administration, or at least the secretary, has political revenge not only against vaccines but against Marana in general.”
Koler said this step will put the United States behind other countries in biomedical research and send a “clear message” to scientists and industry that it is not wise to invest in the technology of the messenger of the Mercy Rana because if they do so, they are likely not to receive funding or approval from the federal government.
Jennifer Nuzu, a professor of epidemics and director of the Wasba Center at the College of Public Health at the University of Brown, told the Hill newspaper that the cancellation represents a threat to national security, as it opens the United States to future public health emergencies caused by the biological war.
“One of the ways we respond to occurring is to say that the United States is completely committed to preparing,” Nuzu said. “When we take them out of the table and leave nothing in their place, we mainly refer to our opponents that we are no longer interested in defending ourselves.”
In the long run, Nuzzo said, the end of research efforts on flexible vaccine platforms will suffocate medical innovation that comes out of the United States, including new treatments for diseases such as cancer.
“It’s worrying about a number of fronts,” she said.
Frank vaccines work by sending instructions to the cells to make a specific type of protein on the virus outer membrane. Then the body’s immune system is able to perceive this protein as a foreign thing and will create antibodies to fight them.
The same general approach to help vaccinate a person can be used against a specific cancer, according to Nuzzo.
“The cancers have a genetic signature,” she said. “We don’t know what will happen, but there are some initial studies that indicate that they may be a very promising way to treat cancer.”
None of the canceled contracts deal directly with cancer research, but this step is likely to cause a chilling effect on the researchers that ultimately affect the work related to cancer, said Michael Ostohm, the founding director of the Research and Policy Research Center in the vaccine safety project.
“I think you will see anyone ready to invest in the technology of the Merseuled Rana, and it will decline, if not completely out of this work,” he said.
“This is very unfortunate, given that we have a number of other infectious diseases that this pollen has been explored in terms of assuming other infectious diseases including some cancer vaccines.”
Kennedy is very skeptical of vaccines in general, and in particular we vaccies.
In 2021, he falsely said that the Covid-19 vaccine was “the most bloody vaccine ever.” Kennedy also faces pressure from his fellow anti -vaccine activists not to do enough to maintain flexible vaccines outside the market.
It was criticized earlier this year when the Food and Drug Administration (FDA) agreed to the updated Covid-19 vaccine from Moderna, although the agency limited its use in children.
In a video posted on X on Tuesday, Kennedy falsely claimed that flexible shots do not protect against respiratory viruses and do not work if a virus is mutating.
“One boom and the vaccine become ineffective,” Kennedy said.
After this announcement, public health experts urged Congress to regain funding for flexible vaccine research, as some described it as a “assault” on the federal policy.
“The HHS secretary continues an indifference to the policy of sound federal vaccines,” said Robert Steinbrok, director of the General Citizen Health Research Group.
“A flexible vaccine platform was necessary to develop and publish the quick vaccine during the CovivD-19 pandemic and is still necessary to prepare for future health emergency cases in the future.”
But the full range of Kennedy’s step is unknown.
A Moderna The Hill spokesman told that they were not aware of any new award since their contract was canceled to help develop the H5N1 Bird Flu in May.
Gritstone Bio, another company in the HHS list of canceled contracts, stopped the operations earlier this year after the company applied for bankruptcy in 2024.
A Tiba Biotech spokesman said the HHS decision to end a cold contract “comes as a surprise” because his contract was for treatment, not a vaccine, and he does not use MRNA technology. Instead, it uses a method called RNA Internet, which FDA agreed to treat some diseases in 2018.