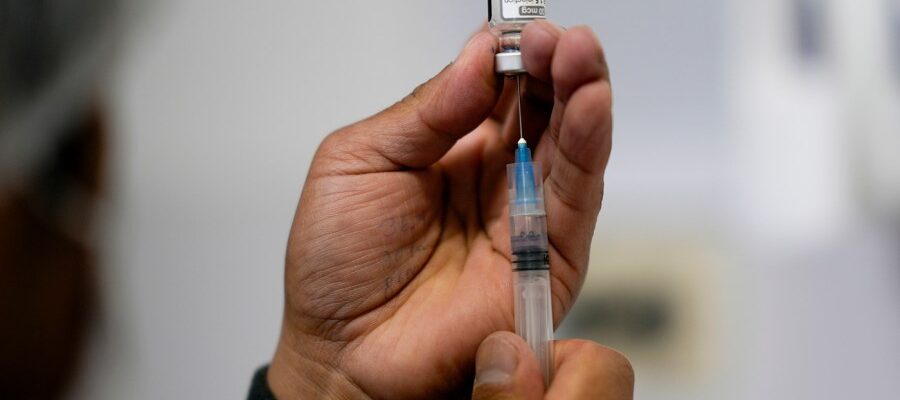
FDA may make it difficult to get young children

Food and Drug Administration (FDA) is considering nullifying a Pfizer Covid-19 vaccine for healthy children under the age of 5, a step that will add another barrier to parents who want to vaccinate healthy children before the respiratory virus season.
Fayzer student is three doses for children between the ages of 6 months and 5 years ago, who has long been available under emergency use (EUA). But according to the company, the Food and Drug Administration weighs this license.
“We are currently in discussions with the agency on the potential tracks forward and we asked that the European Union to remain this age group in its place for the 2025-2026 season,” Pfizer told Hill.
The company added: “It is important to note that these deliberations are not related to the safety and effectiveness of the vaccine, which still shows a positive profile.”
Pfizer expects the Food and Drug Administration (FDA) to approve its vaccine for children between the ages of 5 and 11 with the same restrictions as Moderna and Novavax.
The FDA (FDA) has agreed in July, on a 6-month Moderna shot for 6 months and above, but only if they have a healthy state of increased risk of severe Covid-19. Novavax has not been approved for children under the age of 12.
PFIZER has fully approval from the Food and Drug Administration on the Covid-19 vaccine for individuals between the ages of 12 years and over since 2022.
It is not unusual for the FDA to cancel the emergency license to the product whether the competitor gains approval to an alternative, and PFIZER has not applied after fully approval to the Covid-19 vaccine for the youngest age group.
All Covid-19 vaccines were only available in light of the emergency use licenses-effective special permissions that were served during the state of emergency if there were no approved alternatives from the Food and Drug Administration.
But given the restrictions imposed on ModeRa shots, if Pfizer permission is withdrawn, healthy children under the age of 5 will not have official options if their parents want to vaccinate them.
“Parents were already struggling. We had fathers, until last year, they had to lead two to three hours to get [COVID] Vaccines. Indeed, access was a problem. Fatima Khan, co -founder of the group, who calls for access to vaccines for children, said.
“You are giving up children at the moment … This is a very safe and very easy for them to manage our children. Why can’t we get it?” Khan said.
In a hill statement, the Ministry of Health and Humanitarian Services (HHS) said it will not comment on the possible changes.
“The Federal Public Health Emergency has ended in May 2023. We do not comment on potential organizational changes. Unless it is officially announced by HHS, the discussion on the future agency conducting pure speculation must be considered,” HHS spokesman Andrew Nixon said.
In May, World Health Minister Robert F. said. Kennedy Junior The CDC and Covid-19 vaccines no longer recommend Covid-19 vaccines for healthy children or pregnant women.
Then the Disease Control Center updated its fortification schedule to reflect that children who have no basic health condition “may receive” Covid-19 vaccines after consulting with the health care provider.
Kennedy expressed what expresses personal hatred towards Moderna and PFIZER footage, describing them as dangerous and unreliable. HHS has just announced that it was retreating from the financing research that involves the MRNA technology that was used to develop vaccines in record time.
State health officials said they were told that Moderna is intensifying its vaccine supply to fall and they will be able to fulfill the demand if the PFIZER vaccine is no longer available for the youngest children. Service providers who have already requested Pfizer shots for the next season can be able to shift to Moderna.
But since the Moderna vaccine is only licensed to children at risk of severe injury, pediatricians will have to provide them with a “external sign” for healthy children without ensuring that it will be covered with insurance.
“We hope that they will be made clinical decisions to request the vaccine, obtain it, and cover it with insurance. But it is very complicated,” said Claire Hanan, Executive Director of the Evance Directors Association, which represents government and local officials.
Hanan said: “The Food and Drug Administration (FDA) came out outside the recommendation process in the way in which the Moderna vaccine was licensed, and therefore there is a lot of ambiguity, and we are just waiting for the direction from the Center for Disease Control.”
Infectious pathologists, and the data of the Disease Control Center shows that children under the age of 2 are at risk of severe Covid-19 even if they are in good health.
Ayana Bennett, director of the Ministry of Health in Colombia, said healthy children should be able to live their best lives. She said that if the vaccination will prevent them from developing a serious disease, it should not be a question.
“I want children not to miss two weeks from school. I want the children not to be transferred to the hospital, or in urgent and miserable care. We want them to be able to live in their lives when there is no reason they should not.” “If I can prevent something, I must do that, and this is something that we have to prevent at all.”